钎焊基本原理
您遇到钎焊方面的问题吗?或需要我们推荐合适的钎焊产品吗?欢迎和我们联系!
鲁科斯钎焊材料(苏州)有限公司
中国 江苏省苏州市苏州工业园区星龙街428号苏春工业坊标准厂房8B
手 机:18862195523
Email:ABian@lucasmilhaupt.com WeChat:bxc615
手 机:13580355062
Email: KCheng@lucasmilhaupt.com
手 机:18660285329
Email:Lli@lucasmilhaupt.com
手 机:13512375105
Email:WWei@lucasmilhaupt.com
电话:15862440316
Email:Jxia@lucasmilhaupt.com
电 话:0512-62891510-215
Email:CFang@lucasmilhaupt.com
Why Brazing Requires Flux?
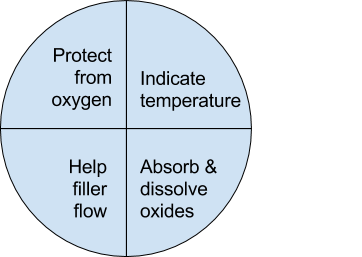
Flux is a chemical compound applied to the joint surfaces before brazing. Its use, with a few exceptions, is crucial in the atmospheric brazing process. Heating a metal surface accelerates the formation of oxides, the result of chemical combination between the hot metal and oxygen in the air. If you don't stop these oxides from forming, they'll inhibit the brazing filler metal from wetting and bonding to the surfaces.
A coating of flux on the joint area guards the surfaces from the air, preventing oxide formation. It also dissolves and absorbs any oxides that form throughout heating or that were not completely removed in the cleaning process.
Understanding the functions and stages of flux will help you achieve strong, quality joints in your operation.
Flux Application
You can apply flux as long as you cover the joint surfaces completely. Flux traditionally is made in a paste, so it's usually most convenient to brush it on. But as production quantities increase, it may be more effective to apply the flux by dipping or dispensing a pre-measured deposit of high-viscosity dispensable flux from an applicator gun. Why dispensable flux? Many companies find the repeatable deposit size improves joint consistency, and because typically less flux is used, the amount of residue entering the waste stream is also reduced.
When do you flux and how do you choose?
Typically just before brazing, if possible. That way the flux has least chance to dry out and fake off, or get knocked off the parts in handling. Which flux do you use? Choose the one formulated for the specific metals, temperatures and conditions of your brazing application. There are fluxes formulated for practically every need; for example - fluxes for brazing at very high temperatures (in the 2000°F/1093°C area), fluxes for metals with refractory oxides, fluxes for long heating cycles, and fluxes for dispensing by automated machines.
The addition of metallic boron changes white flux to black. Black flux is beneficial for fast induction heating, may provide better protection in a hightemperature brazing operation, and can be helpful with high-liquidus filler metals.
Boron-modified black flux can help ensure a successful braze joint when brazing large parts over an extended period of heating time, or with base materials that require extra care to reduce tenacious oxides. Lucas Milhaupt's technical staff demonstrates the difference between white versus black flux in this video.
Fortunately for your inventory, general-purpose fluxes, such as Handy & Harman's Handy Flux, are suitable for most typical brazing jobs.
How much flux do you use?
Enough to last throughout the entire heating cycle. Keep in mind that the larger and heavier the pieces brazed, the longer the heating cycle will take - so use more flux. (Lighter pieces, of course, heat up faster and require less flux.) As a general rule, don't skimp on the flux. It's your insurance against oxidation. Think of the flux as a sort of blotter. It absorbs oxides like a sponge absorbs water. An insufficient amount of flux will quickly become saturated and lose its effectiveness. A flux that absorbs less oxides not only insures a better joint than a totally saturated flux, but it is a lot easier to wash off after the brazed joint is completed.
Flux can also act as a temperature indicator, minimizing the chance of overheating the parts. Handy & Harman's Handy Flux, for example, becomes
completely clear and active at 1100°F/593°C. At this temperature, it looks like water and reveals the bright metal surface underneath - telling you that the base metal is just about hot enough to melt the brazing filler metal.
Fluxing is an essential step in the brazing operation, aside from a few exceptions. You can join copper to copper without flux, by using a brazing filler metal specially formulated for the job, such as Handy & Harman's Sil-Fos or Fos-Flo 7. (The phosphorus in these alloys acts as a fluxing agent on copper.) You can also omit fluxing if brazing occurs in a controlled atmosphere (i.e. a gaseous mixture contained in an enclosed space, usually a brazing furnace). The atmosphere (such as hydrogen, nitrogen or dissociated ammonia) completely envelops the assemblies and, by excluding oxygen, prevents oxidation. Even in controlled atmosphere brazing you may find that a small amount of flux improves the wetting action of the brazing filler metal.
